
 Location:Home/News/Company News
Location:Home/News/Company NewsThe 2023 CSCO Guidelines Conference, co-hosted by the Chinese Society of Clinical Oncology and the Beijing Xisike Clinical Oncology Research Foundation, commenced on April 21, 2023. This event featured updates to the guidelines for major tumor types and in-depth interpretations of relevant contents, serving as a foundation for clinical practice and standardized diagnosis and treatment.
In the updated guidelines for the treatment of advanced melanoma, the evidence level for the MEK inhibitor Tunlametinib Capsules has been elevated to a Second Line-Level 1 recommendation. The updates are as follows:
Advanced Skin Melanoma:
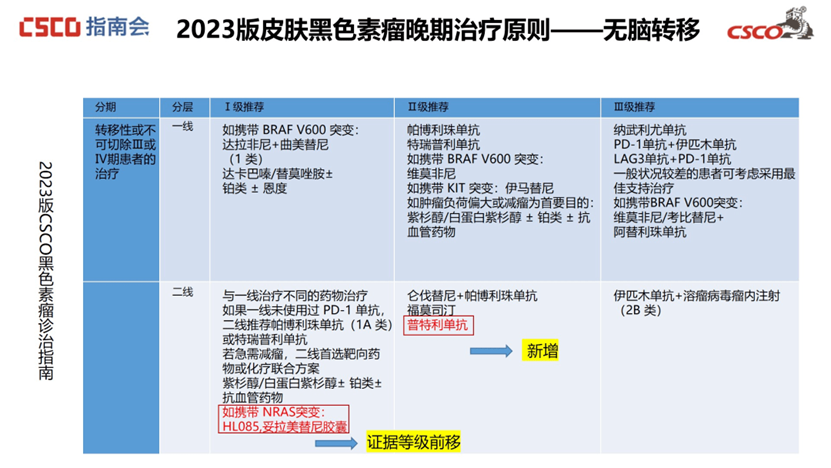
For patients without brain metastases carrying NRAS mutations, Tunlametinib Capsules (HL-085) are now recommended as a Level 1 treatment, upgraded from the previous Second Line-Level 3 recommendation.

For patients with brain metastases carrying NRAS mutations, Tunlametinib Capsules (HL-085) are now recommended as a Level I treatment, also upgraded from the prior Second Line-Level 3 recommendation.
Advanced Acral Melanoma:
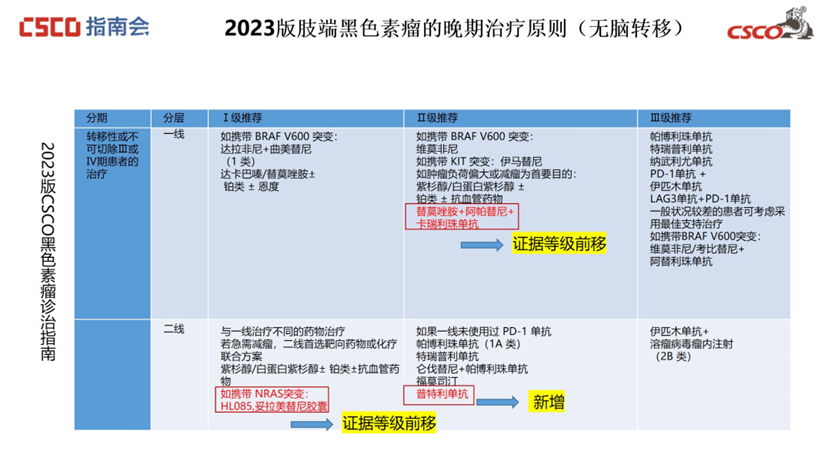
For patients without brain metastases carrying NRAS mutations, the recommendation for Tunlametinib Capsules (HL-085) has been upgraded from Second Line-Level 3 to Level 1 recommendation.

For patients with brain metastases carrying NRAS mutations, Tunlametinib Capsules (HL-085) similarly received an upgrade from Second Line-Level 3 to Level 1 recommendation.
Advanced Mucosal Melanoma:
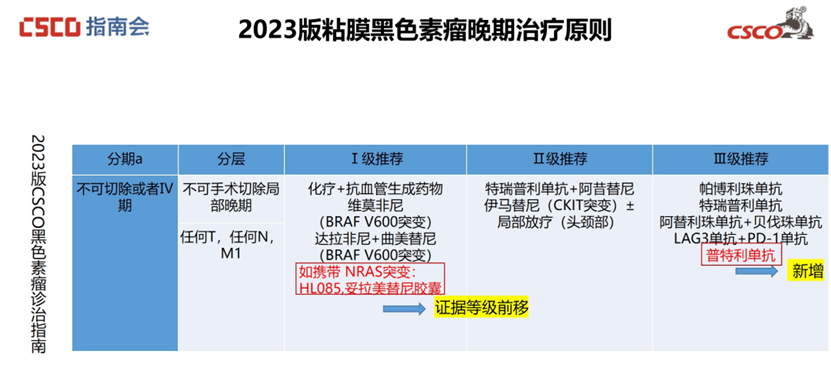
For patients with unresectable or stage IV mucosal melanoma carrying NRAS mutations, the recommendation for Tunlametinib Capsules (HL-085) has moved from a Level 3 to a Level 1 recommendation.
About KeChow Pharma
KeChow Pharma is dedicated to developing innovative treatments for tumors, free radical-related diseases, and other therapeutic areas. Its pipeline includes MEK inhibitor, chemoprotective agents, KRASG12C inhibitor, pan-RAF inhibitor, and multiple other tumor-targeted inhibitors. Notably, its MEK inhibitor, Tunlametinib (HL-085), has submitted a new drug application in China and is included in the CSCO guidelines. Another novel drug, a chemoprotective agent, demonstrates superior efficacy and safety compared to current clinical counterparts. All research efforts aim to address unmet clinical needs globally and within China by developing original small molecule drugs with “best-in-class” potential, bridging domestic gaps and fulfilling scientific aspirations to enhance quality of life.