
 Location:Home/News/Company News
Location:Home/News/Company NewsThe first worldwide MEK inhibitor approved for treating advanced NRAS-mutant melanoma.
The first MEK inhibitor independently developed in China.
Recognized for its best-in-class potential, demonstrating an ORR of 34.7% in a pivotal Phase II trial.
The 2023 CSCO Melanoma Diagnosis and Treatment Guidelines recommends Tunlametinib as a Level 1 therapy for advanced NRAS-mutant melanoma.
Today, the official website of the National Medical Products Administration (NMPA) announced the approval of Tunlametinib (trade name: Kolupin) for marketing. Developed by KeChow Pharma, this Class 1 small molecule innovative drug is intended for patients with advanced NRAS-mutant melanoma who have not responded to PD-1/PD-L1 treatments.
 (Approval document)
(Approval document)
The approval is based on a single-arm, multi-center pivotal Phase II registration study, which included 100 patients to evaluate the efficacy and safety of Tunlametinib (HL-085) in the treatment of patients with advanced NRAS-mutant melanoma. According to the independent review committee, as of February 19, 2023, the confirmed objective response rate (ORR) was 34.7% (see Figure 1), with a median progression-free survival (mPFS) of 4.2 months and a 1-year overall survival (OS) rate of 57.2%. Notably, the ORR for patients who had previously undergone immunotherapy reached 39.1%. The most common Level 3 or higher treatment-related adverse events included increased blood creatine kinase, diarrhea, and edema. Overall, the safety profile is considered controllable and manageable, with no study drug-related deaths reported during the trial.
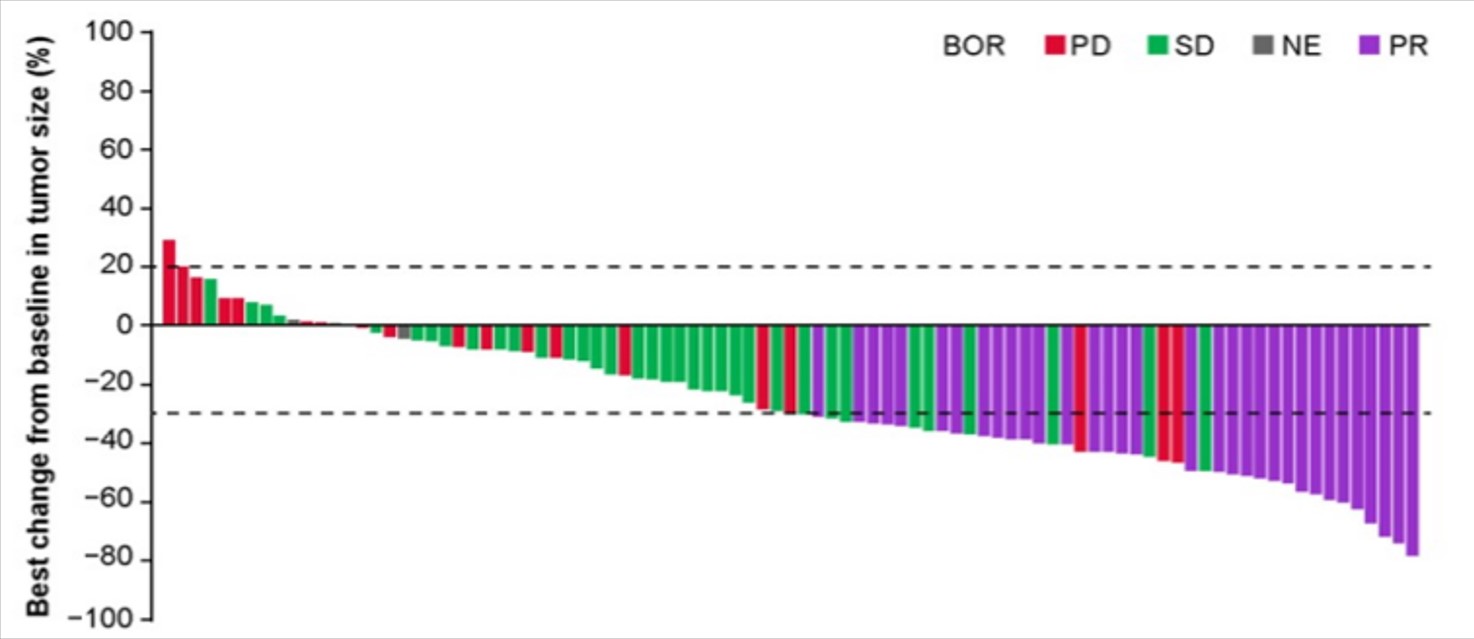
Figure 1. Waterfall Chart of Lesion Percentage Change
In the 2023 edition of the CSCO Melanoma Diagnosis and Treatment Guidelines[1], the evidence level for Tunlametinib in treating advanced melanoma has been elevated.
For patients with advanced skin and acral melanoma, regardless of brain metastases, Tunlametinib (HL-085) is now recommended as a First Line treatment for those with NRAS mutations, upgraded from the previous Second Line-Level 3 recommendation to a Level 1 recommendation.
Similarly, for patients with unresectable or stage IV mucosal melanoma carrying NRAS mutations, Tunlametinib (HL-085) has also moved from a Level 3 recommendation to a Level 1 recommendation.
Currently, a confirmatory Phase III randomized controlled trial of Tunlametinib (HL-085) for advanced NRAS-mutant melanoma is underway. Additionally, key registration clinical studies are in progress to evaluate Tunlametinib (HL-085) in combination with BRAF inhibitor for treating BRAF V600-mutant metastatic non-small cell lung cancer and metastatic colorectal cancer. Upon approval, Tunlametinib (HL-085) is expected to benefit a broader range of patients with solid tumors harboring RAS and RAF mutations.
About NRAS-mutant melanoma:
Malignant melanoma (MM) is a highly malignant tumor originating from epidermal melanocytes or nevi, primarily occurring in the skin and ranking third among skin malignancies [2]. In China, there are approximately 20,000[3] new cases of melanoma annually, with the median age of onset between 50 and 69 years [3]. Recently, the incidence has been rising rapidly, increasing the disease burden each year. Notably, there are significant differences in melanoma types between China and other countries. In Europe and the U.S., about 90% of cases are of the cutaneous type, which tends to have a relatively better prognosis. In contrast, in China and Asia, the more malignant acral and mucosal types predominate, accounting for approximately 50% and 20-30% [4] of cases, respectively. NRAS-mutant melanoma is a particularly aggressive and fast-progressing subtype, comprising about 10.4-12.6% [5] of all melanoma cases. This subtype shows low sensitivity to immunotherapy and chemotherapy, with studies indicating that the overall objective response rate (ORR) to anti-PD-1 treatments in Asian NRAS-mutant melanoma patients is around 6.1% [6]. Currently, no targeted drugs have been globally approved for this subtype, highlighting an urgent need for the development of effective anti-tumor therapies.
About Kolupin
Kolupin (Tunlametinib/HL-085) is a highly selective MEK inhibitor independently developed in China. It targets MEK1/2 in the RAS-RAF-MEK-ERK signaling pathway, effectively blocking downstream signal transmission and inhibiting tumor growth. Kolupin is intended for patients with advanced NRAS-mutant melanoma who have not responded to anti-PD-1/PD-L1 therapies. The recommended dosage is 12 mg, administered orally twice a day (approximately every 12 hours), either on an empty stomach or with meals.
About KeChow Pharma
KeChow Pharma is dedicated to developing innovative treatments for tumors, free radical-related diseases, and other therapeutic areas. The Company boasts a robust pipeline, highlighted by the MEK inhibitor Kolupin (Tunlametinib/HL-085), which has received marketing approval in China. KeChow’s research initiatives target unmet clinical needs in China and globally, focusing on creating novel, best-in-class small molecule drugs to fill gaps in the market. KeChow aims to fulfill the high expectations for scientific advancements and improve quality of life worldwide.
Reference
1. 2023 CSCO guideline for the diagnosis and treatment of melanoma in China
2.ZAREMBA A, ZIMMER L, GRIEWANK K G, et al. Immunotherapy for malignant melanoma[J]. Internist (Berl), 2020, 61(7): 669-675.
3. Research white paper on the behavior status of melanoma patients in China.
4. 2022 Chinese guideline for diagnosis and treatment of melanoma
5.Min Ren , Jing Zhang, Yunyi Kong, et al. BRAF, C-KIT, and NRAS mutations correlated with different clinicopathological features: an analysis of 691 melanoma patients from a single center. Ann Transl Med. 2022 Jan;10(2):31.
6.Li Zhou, Xuan Wang, Zhihong Chi, et al. Association of NRAS Mutation With Clinical Outcomes of Anti-PD-1Monotherapy in Advanced Melanoma: A Pooled Analysis of Four Asian Clinical Trials. Front Immunol. 2021 Jul 5:12:691032